Patient Safety, Brand Reputation
Single-use influences all of the touchpoints — patient safety, cost effectiveness, COVID-19, and convenience —that factor into building and protecting a sterling healthcare brand. Healthcare reputation management, after all, isn’t solely limited to crisis communications. It’s important to detect vulnerabilities early and prevent any incidents that might harm a hospital’s quality of care.
Read more:
A patient is admitted to the hospital with all hope and expectation of getting better — but instead ends up getting sicker, possibly with a potentially deadly “superbug” infection.
That exact scenario — which happens more than we think — faced three U.S. hospitals in the last decade, when a cluster of antibiotic-resistant infections were traced to a duodenoscope, used to diagnose and treat diseases of the pancreas and bile ducts.
In the fall of 2014, an outbreak of carbapenem-resistant Enterobacteriaceae (CRE) at one hospital was linked to duodenoscopes; more than 180 patients would be warned of possible contamination. Seven were infected and two, already seriously ill, died.
At another hospital, 39 people were infected by contaminated scopes, and 18 of them died.
Seventeen months after that first outbreak, endoscope manufacturers and the U.S. Food and Drug Administration began alerting hospitals, doctors and the public to the risks of cross-contamination from scopes that aren’t fully cleaned of harmful bacteria.
Exhaustive investigations ensued in hopes of pinpointing the source of the elusive superbug. Lawsuits were filed against endoscope manufacturers; the manufacturers cast blame on the hospitals for not following the cleaning instructions — a complex sequence of more than 100 steps that can take hours to complete.
The national repercussions reverberated. Unceasing media scrutiny followed. Hospital executives were forced to enact crisis communications plans. Ongoing litigation brought even more scrutiny of patient safety practices.
‘Undetected for Far Too Long’
A federal investigation would later conclude that “future device issues are likely to go undetected for far too long and with life-threatening consequences” absent a “comprehensive postmarket device surveillance system that supplements self-reporting from hospitals and manufacturers.”
A report, issued in January 2016 by the U.S. Senate’s Health, Education, Labor, and Pensions Committee, sketched out five key recommendations, including making adverse event reporting related to medical devices a condition for hospitals to participate in Medicare.
What it also did was underscore how sterling healthcare brands can be exposed to the kind of reputational and financial risk that can damage a hospital system’s hard-fought track record in patient safety and satisfaction.
Healthcare reputation management, after all, isn’t solely limited to crisis communications. It’s important to detect vulnerabilities early and anticipate and prevent any incidents that might do harm to a hospital’s quality of care.
That’s because brand loyalty is a key performance indicator for healthcare organizations. Consumer loyalty can enhance profitability, by increasing recurring revenue and driving down costs. And yet, 2021 consumer trends research by healthcare performance improvement firm NRC Health shows that brand indifference is rising among healthcare consumers: More than one-third of consumers (36 percent) say they have no particular preference for a healthcare brand.
Convenience plays a key role. NRC Health asked consumers to list their reasons for choosing a healthcare provider and almost half (49 percent) listed “convenient locations” as the primary driver. Fifty-two percent said convenience is their second-most important factor in choosing a healthcare brand.
“The message for healthcare organizations, then, is clear,” NRC Health writes. “Organizations have, by and large, done outstanding work in offering highly satisfying care experiences, in spite of the pandemic. Going forward, it may be prudent to consider measures that improve the speed and ease with which consumers can access those experiences.”
COVID-19 has only accelerated these trends. Six in 10 now say they expect their trusted healthcare brands to change once the pandemic has ended and 45 percent indicate those preferences have already evolved.
Patient safety has obvious and far-reaching repercussions on how hospital brands are viewed. Did you know the third-leading cause of death in the U.S. is preventable errors, accidents and infections at hospitals, totaling an estimated 20,000 fatalities per month? Press Ganey, a healthcare quality researcher, says that after a two-year period of improvement, “safety culture” scores declined sharply at a sampling of 54 hospitals across the country in 2020.
Perhaps even more worrisome, a separate survey of 160 hospitals by the Agency for Healthcare Research and Quality found a 40 percent drop from 2018 to 2020 in staff perceptions that management made safety a top priority.
“Before the pandemic, the gradual trend in safety culture themes was worrisome: Improvements in safety culture weren’t happening fast enough. When COVID-19 hit, it amplified the already high-pressure environment at the nation’s health care organizations, and safety culture outcomes suffered,” Press Ganey writes.
Getting to Zero Harm
Single-use influences all of these touchpoints — patient safety, worker safety, cost effectiveness, COVID-19, and convenience.
After the release of the Senate report into the superbug outbreaks, the FDA mandated that post-market surveillance contamination studies be conducted by reusable endoscope manufacturers Olympus, Fujifilm, and Pentax. The studies found that approximately 6.8 percent of samples taken from reusable duodenoscopes were contaminated, with 5 percent of samples containing high-concern organisms such as E. coli and Pseudomonas aeruginosa.
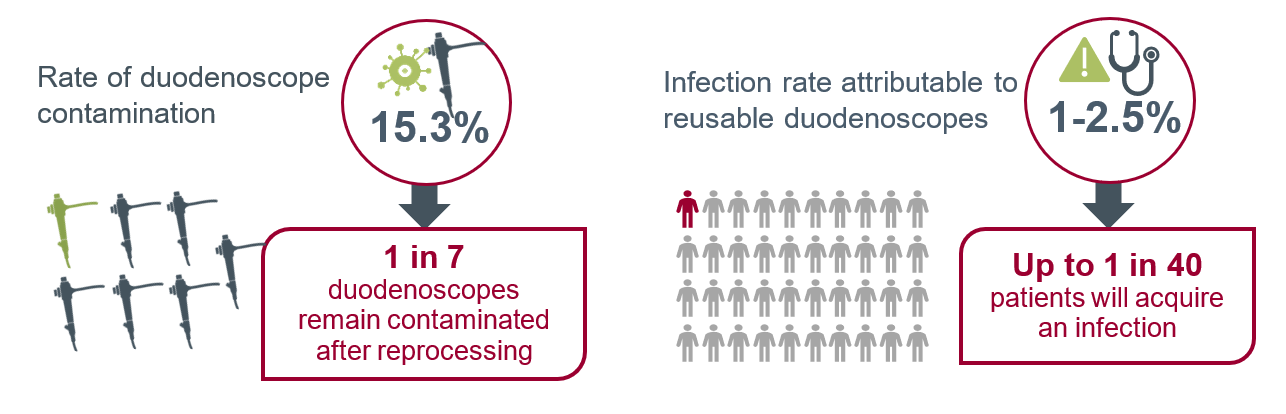
Studies completed by other researchers have found even higher contamination rates. A systematic review and meta-analysis found the contamination rate of all patient-ready duodenoscopes to be 15.3 percent. Scopes that only underwent HLD were found to have a contamination rate of 16.14 percent; the contamination rate was 9.2 percent for endoscopes that underwent either double high-level disinfection (HLD) or ethylene oxide (EtO) gas sterilization.
When it comes to cost, it’s easy to assume that single-use will be more expensive than reusables. For many facilities, however, single-use is cost neutral or even saves money. When all the benefits of single-use are considered — including streamlining operations, improving workflow and efficiency, protecting healthcare professionals (particularly reprocessing technicians), and reducing cross contamination and patient infection risk — it is the cost-effective solution.
The disconnect? Physician, supply chain, operations, and finance leaders often do not have a full picture of all the cost drivers associated with using reusable endoscopes. Factor in eliminating reputational and financial risk from lawsuits and ensuring compliance with The Joint Commission’s “zero harm” accreditation standard, and single-use is the better, safer and smarter alternative to reusables.
And it can help fortify a hospital’s patient safety reputation at a time when, a second year into the novel coronavirus pandemic, conversations around healthcare quality are playing out against a wholly different backdrop. Those deliberations are now analyzed through the prism of access to care, standards and disparities, and innovative approaches to infection control.
Federal regulators, meanwhile, continue to scrutinize reports of infections associated with reprocessed endoscopes. The U.S. Food and Drug Administration announced April 1, 2021, that it is investigating a number of medical device reports detailing patient infections and other contamination issues possible associated with reprocessed urological endoscopes, including cystoscopes, ureteroscopes, and cystourethroscopes.
The FDA said it received more than 450 MDRs over a four-year span and is “very concerned” about three reported deaths (all outside of the U.S.) linked to these infections.
Press Ganey writes in its safety culture research that as the U.S. cautiously moves out of pandemic crisis mode, “reinvigorating safety culture should be top of mind at health systems to yield the best possible outcomes for patients and caregivers. … Leadership needs to message on safety and verbally and visibly acknowledge that protecting patients and the workforce from harm — getting to Zero Harm — is an uncompromisable precondition of every action at the organization.”
What’s stopping your organization from making single-use endoscopy part of its safety culture?
Single-Use Endoscopy
Check out the latest in endoscopy research and best practices at this learning center by Ambu USA.
There is overwhelming evidence that a significant portion of reusable endoscopes remains contaminated with harmful organisms after thorough reprocessing. Studies show that even when reprocessing guidelines and manufacturers’ instructions for use (IFUs) are followed, contamination still occurs in about 15 percent of duodenoscopes and bronchoscopes. 1,2 This contamination results in high rates of infection in patients.
There are multiple reasons why reusable endoscopes are so challenging to disinfect, including human factors and the design of the endoscope itself. The only way to prevent contamination and infection is to move to single-use endoscopes.
Duodenoscopes Under the Microscope
 After multiple reports of an outbreak of deadly infections linked to reusable duodenoscopes in 2016, the U.S. Senate Health, Education, Labor, and Pensions (HELP) Committee launched an investigation. 3,4 The committee concluded that duodenoscopes are a significant patient safety concern and asked the Food and Drug Administration (FDA) to further evaluate the safety of duodenoscopes.5
After multiple reports of an outbreak of deadly infections linked to reusable duodenoscopes in 2016, the U.S. Senate Health, Education, Labor, and Pensions (HELP) Committee launched an investigation. 3,4 The committee concluded that duodenoscopes are a significant patient safety concern and asked the Food and Drug Administration (FDA) to further evaluate the safety of duodenoscopes.5
After the release of the Senate report, the FDA mandated that post-market surveillance contamination studies be conducted by the reusable manufacturers Olympus, Fujifilm, and Pentax. The studies found that approximately 6.8 percent of samples taken from the reusable duodenoscopes were contaminated, with 5 percent of samples containing high-concern organisms such as E. coli and Pseudomonas aeruginosa.6,7
Studies completed by other researchers have found even higher contamination rates. A systematic review and meta-analysis found the contamination rate of all patient-ready duodenoscopes to be 15.3 percent.8 Scopes that only underwent HLD were found to have a contamination rate of 16.14 percent; the contamination rate was 9.2 percent for endoscopes that underwent either double high-level disinfection (HLD) or ethylene oxide (EtO) [KJ1] gas sterilization.8
A Stanford University study, meanwhile, found the associated infection rates for duodenoscopes to be 1 percent, 1.5 percent, and 2.5 percent for HLD, double HLD, and EtO sterilization, respectively. 9
Based on the results of the post-market surveillance contamination study, the FDA released a safety communication in April 2020 recommending that providers transition to fully disposable duodenoscopes such as the Ambu aScope Duodeno or duodenoscopes with disposable parts, although the agency noted that partially disposable scopes retain cross-contamination risks.10
Bronchoscopes and ‘Superbugs’
Similar to duodenoscopes, contamination and infection from bronchoscopes is higher than many expect. A systematic literature review and cost analysis of 16 studies published in the journal Anesthesia in 2020 found a bronchoscope contamination rate of 15.2 percent and the risk of patient infection post-bronchoscopy to be 2.8 percent.1
In the study, a total of 476 contaminations were found in 3,120 procedures.1 Of those contaminated, 86 patients were reported to have a bronchoscope-related infection such as pneumonia.1
Additionally, concerns about multidrug-resistant organisms (MDROs), or superbugs, have sparked worry among many bronchoscopists about the effectiveness of current reprocessing practices. A 2020 literature review found that bronchoscopes may pose an underrecognized potential for transmission of superbugs.11 The study found that contamination risk remains even when cleaning and reprocessing are performed correctly. The evidence is clear that the only way to eliminate patient infections resulting from bronchoscopy is by transitioning to single-use bronchoscopes.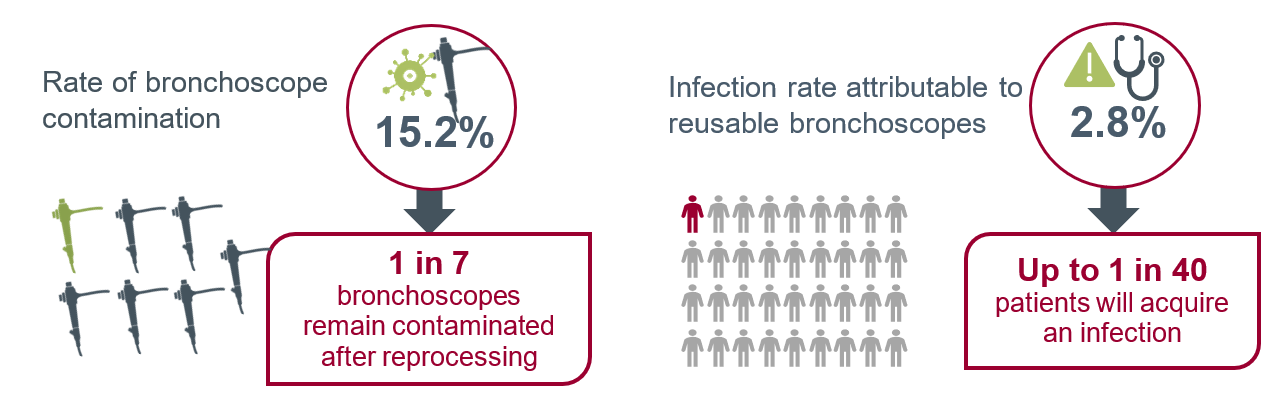
What Drives Contamination and Infection Rates?
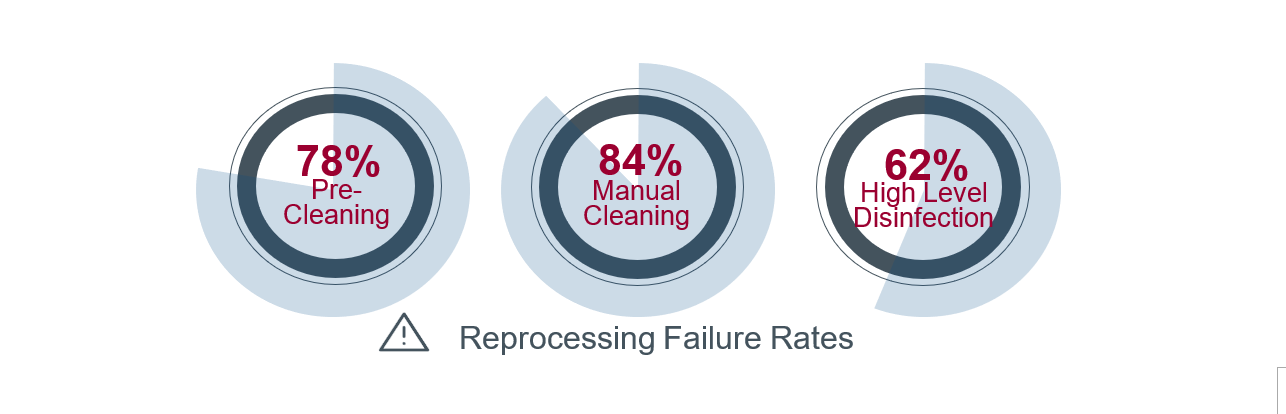
The most common factors associated with microbial transmission include inadequate cleaning, disinfection, and drying procedures. The FDA surveillance studies of reusable duodenoscopes found very high reprocessing failure rates. Across the studies, 78 percent of pre-cleaning steps, 84 percent of manual cleaning steps, and 62 percent of HLD steps were either not performed or performed incorrectly.6,7
 Beyond reprocessing challenges, endoscope design and maintenance issues also result in high contamination rates. Reusable endoscopes cannot be heat sterilized and have multiple channels, which are very difficult to completely disinfect. Additionally, damage such as scratches and other imperfections in the scope itself can foster bacterial growth. In these areas of the scope, bacteria can form biofilms, which are very difficult to eliminate.13
Beyond reprocessing challenges, endoscope design and maintenance issues also result in high contamination rates. Reusable endoscopes cannot be heat sterilized and have multiple channels, which are very difficult to completely disinfect. Additionally, damage such as scratches and other imperfections in the scope itself can foster bacterial growth. In these areas of the scope, bacteria can form biofilms, which are very difficult to eliminate.13
Preventing Patient Infection
Studies have shown that reprocessing failure rates are very high. Additional training and education are needed to increase understanding of the risks and to promote adherence to guidelines and recommendations. However, research suggests that even when reprocessing is performed 100 percent correctly, some endoscopes will remain contaminated and pose a risk to patients.
Given these insights, and the inherent endoscope design issues that promote microbial growth and biofilm formation, the only way to complete eliminate the patient infection risk resulting from use of bronchoscopes is to utilize single-use endoscopes.
References:
1 Mouritsen, J m et al., A systematic Review and Cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes, Anaesthesia 2020, 75:529-540
2 Larsen S. Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. The Lancet. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(20)30195-4/fulltext. Published July 14, 2020.
3 Aleccia et al. “More Infections From Dirty Scopes, Sen. Murray Investigations Finds.” Seattle Times. January 2016
4 Brady Dennis, “Tainted Medical Scopes That Killed 2 in Chicago Area Have Sickened Hundreds in U.S. Europe: Senate Probe.” Chicago Tribune. January 2016
5 Senator Patty Murray, “Preventable Tragedies: Superbugs and How Ineffective Monitoring of Medical Device Safety Fails Patients.” United States Senate Health, Education, Labor, and Pensions Committee. January 2016
6 Olympus Sampling and Culturing Study, FDA 522 Postmarket Surveillance Studies Database, #PS150003. FDA 2019. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=354&c_id=3726
7 Pentax Sampling and Culturing Study, FDA 522 Postmarket Surveillance Studies Database, #PS150004. FDA 2019. Available at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=355&c_id=3727
8 Larsen S. Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. The Lancet. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(20)30195-4/fulltext. Published July 14, 2020.
9 Barakat, et al, 2020. Cost Utility Analysis Comparing Duodenoscope Reprocessing/Sterilization, Novel Duodenoscopes with Disposable Endcaps and Fully Disposable Duodenoscopes. Digestive Disease Week: 775-2020.
10 “The FDA is Recommending Transition to Duodenoscopes with Innovative Designs to Enhance Safety: FDA Safety Communication.” FDA, April 2020
11 A. C. Mehta and L. F. Muscarella. (Feb. 2020). Bronchoscope-Related ‘Superbug’ Infections. Chest, vol. 157, no. 2, pp 454-469. doi:10.1016/j.chest.2019.08.003 LK
Endoscopy Insights Podcast
A new podcast from Single-Use Endoscopy, which showcases conversations with thought leaders in flexible and single-use endoscopy.