The challenges of reprocessing reusable flexible gastrointestinal endoscopes
Inadequate reprocessing may increase the risk of cross-contamination. Studies have investigated the contamination rates of various endoscope types and the results are significant. Explore the contamination challenges posed by reprocessed gastrointestinal endoscopes in this new white paper.
A few facts about patient cross-contamination risks
Get an overview of the main issues around the risk of patient cross-contamination with reusable GI endoscopes. Also learn about the three main challenges related to reprocessing and get facts and figures from patient cross-contamination studies.
The three main challenges associated with GI endoscope reprocessing
Challenge #1
Complex cleaning processes
![]()
Challenge #2
Scope design makes cleaning difficult
![]()
Challenge #3
The rise of the superbugs
![]()
Challenge #1
Complex, resource-demanding, and ultimately ineffective cleaning and reprocessing
Multiple studies have shown that regardless of which guidelines were followed, no cleaning process effectively removes bacteria1,2,3. This has been true whether the reprocessing was performed using high-level disinfection (HLD), double HLD or HLD and sterilization combined4,5.
These steps represent a condensed version of the basic recommended actions. In reality, there can be more than 100 steps6. Individual guidelines should be developed per institution by a multidisciplinary team.
![]()
![]()
Reprocessing steps:
PRE-CLEANING
CRUCIAL FOR AVOIDING BIOFILM
Important
Must be performed after procedure carefully following IFU.
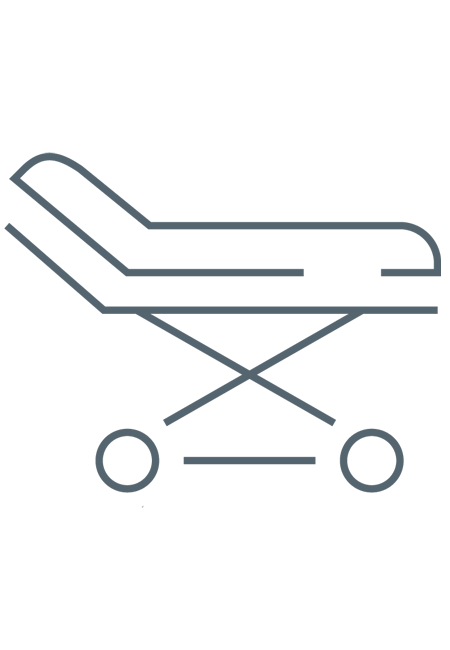
LEAK-TESTING
DETECT INTERNAL/EXTERNAL DAMAGE
Important
Damage can lead to inadequate disinfection & further deterioration.
MANUAL CLEANING
MOST CRITICAL STEP FOR DISINFECTION
Important
Meticulous cleaning is a prerequisite to HLD and sterilization.
VISUAL INSPECTION
ADDITIONAL SAFETY ASSURANCE
Important
The complex design of flexible GI scopes makes it difficult to detect problems.

DISINFECTION OR STERILIZATION
CRITICAL TO ADHERE TO INSTRUCTIONS
Important
The FDA recommends double HLD or sterilization.
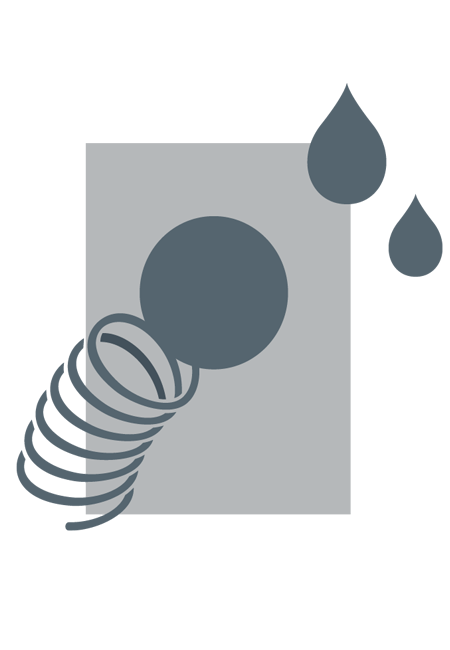
STORAGE
PROTECTS FROM MOISTURE
Important
Incorrect storage can result in bacteria growth.

If the margin of safety for endoscope reprocessing is so small that perfect compliance with >100 pages of the manufacturer’s instructions for use is required, then the endoscope design is too complex, the microbial load is too high, and the process is too unforgiving to be practical in the real world 7.
William A. Rutala, PhD, MPHX
Challenge #2:
The design of the scopes makes cleaning inherently difficult
Difficult-to-reach areas1,8, and deterioration4,5, due to routine use and cleaning make GI endoscopes more susceptible to moisture and bacterial contamination.
Places bugs like to hide
Many GI professionals are aware that the recesses and complex design of the elevator (1) and other intricate parts of the distal tip (2) make duodenoscopes difficult to clean.
However, studies indicate that other parts of GI endoscopes present challenges as well9,10. Bacteria can hide in many places including the biopsy port and working channel (3), the remote switches, the valves and the wheels of the control handle (4). Endoscopic accessories and tools used during routine cleaning may further cause grooves to form in channels, giving bacteria another place to hide (5).
NOTE: The drawing is meant for illustrative purposes only and is not intended to represent any specific endoscope.
Challenge #3:
The rise of the superbugs
It is already challenging to clean endoscopes but the rise in recent years of Multi-Drug Resistant Organisms (MDROs), sometimes referred to as superbugs, have added to the problem. For example, more than 15 reported outbreaks related to MDROs have occurred since 200011.
What is the contamination rate of reusable duodenoscopes used for ERCP?
A meta-analysis of 15 studies (Larsen et al., EClinicalMedicine, 2020) set out to determine an average reference point for the contamination rate of reusable duodenoscopes used for ERCP. Ian Haislip, Associate Health Economist at Ambu, reviews the evidence of the meta-analysis in the video below.
Patient cross-contamination evidence
A growing concern about the risk of patient cross-contamination linked to reusable endoscopes has come into even more focus due to the recent COVID-19 pandemic, the rise of MDROs and many studies like those below.

According to data, 18% of duodenoscopes had a positive culture after initial HLD. (Mark, et al., 2020)
The presence of microorganisms with gastrointestinal or oral origin, independent of CFU count was ~15% for duodenoscopes (Rauwers et al., 2020)
Fifteen patients (14.4%) were infected with carbapenem-resistant Enterobacteriaceae via contaminated duodenoscopes (Kim et al., 2016)

Study showing colonoscopes contaminated with non-tuberculous mycobacteria after reprocessing (Satta et al., 2020).

94 out of 516 (18.5%) duodenoscopes were contaminated. There were no significant differences between sHLD, dHLD, or HLD/ETO for MDRO (Snyder et al., 2017).

59 out of 627 (9.4%) elevator cultures were positive after double HLD (Rex et al., 2018).
References:
1 Rauwers AW, Voor in ‘t holt AF, Buijs JG, de Groot W, Erler NS, Bruno MJ, Vos MC, Nationwide risk analysis of duodenoscope and linear echoendoscope contamination, Gastrointestinal Endoscopy (2020), doi: doi.org/10.1016/j.gie.2020.05.030
2 Rex DK, Sieber M, Lehman GA, et al. A double-reprocessing high-level disinfection protocol does not eliminate positive cultures from the elevators of duodenoscopes. Endoscopy. 2018;50(6):588-596. doi:10.1055/s-0043-122378.
3 Naryzhny I, Silas D, Chi K. Impact of ethylene oxide gas sterilization of duodenoscopes after a carbapenem-resistant Enterobacteriaceae outbreak. Gastrointestinal Endoscopy (2016), doi: dx.doi.org/10.1016/j.gie.2016.01.055
4 Snyder GM, Wright SB, Smithey A, et al. Randomized Comparison of 3 High-Level Disinfection and Sterilization Procedures for Duodenoscopes. Gastroenterology. 2017;153(4):1018-1025. doi:10.1053/j.gastro.2017.06.05 2.
5 Kovaleva J. Infectious complications in gastrointestinal endoscopy and their prevention. Best Pract Res Clin Gastroenterol. 2016;30(5):689-704. doi:10.1016/j.bpg.2016.09.008.
6 Ostead et al., 2017: A glimpse at the true cost of reprocessing endoscopes: Results of a pilot project.
7 Rutula et Al, What’s new in reprocessing endoscopes: Are we going to ensure “the needs of the patient come first” by shifting from disinfection to sterilization?, American Journal of Infection Control 47 (2019).
8 Mark J, Underberg K, Kramer R, Results of duodenoscope culture and quarantine after manufacturer-recommended cleaning process, Gastrointestinal Endoscopy (2020), doi: doi.org/10.1016/j.gie.2019.12.050
9 Ofstead CL, Wetzler HP, Heymann OL, Johnson EA, Eiland JE, Shaw MJ. Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures. Am J Infect Control. 2017;45(2):e26-e33. doi:10.1016/j.ajic.2016.10.017
10 Barakat MT, Girotra M, Huang RJ, Banerjee S. Scoping the scope: endoscopic evaluation of endoscope working channels with a new high-resolution inspection endoscope (with video). Gastrointest Endosc. 2018;88(4):601-611.e1. doi:10.1016/j.gie.2018.01.018
11 Rubin ZA, Kim S, Thaker AM, Muthusamy VR. Safely reprocessing duodenoscopes: current evidence and future directions. Lancet Gastroenterol Hepatol. 2018;3(7):499-508. doi:10.1016/S2468-1253(18)30122-5.
Ambu® aScope™ Duodeno
Helps you address concerns about patient cross-contamination.


