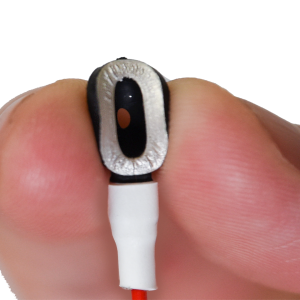
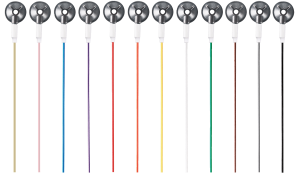MRI EEG Hard Cup Electrodes
Single-use MR conditional cup electrodes provide a high quality recording while eliminating the hassle of cleaning and sanitizing electrodes. A disposable harness cable is included. You may also purchase a reusable harness cable.
Key Benefits
General information
The MR conditional electrodes also save time and money. Leaving the cup electrodes on the patient during an MRI eliminates the need to call in techs during off hours which saves money. The soft cup design as well as eliminating the need to remove the electrodes during an MRI reduce the chances of skin breakdown.
The Ives Disposable Plastic Conductive Electrodes are 510K cleared for use in MR 1.5T and 3.0T environment.
The error free connection is designed to make all electrodes interchangeable. A common harness and connection system make disconnection and reconnection easy, quick and error free.
Specifications
| Dimension | |
|---|---|
| Diameter of cup (in mm) | 10 |
| Height of cup (in mm) | 3 |
| Environment | |
|---|---|
| PVC-free electrode | Yes |
| Latex-free electrode | Yes |
| PVC-free lead wire | No |
| Latex-free lead wire | Yes |
| PVC-free packaging | No |
Spare parts
There are no spare parts or accessories for this product.Downloads
Brochures
file_download Neurodiagnostics Catalog
(8.9 MB - pdf)
(8.9 MB - pdf)
file_download Ambu Single-Use Product Portfolio Brochure
(2.04 MB - pdf)
(2.04 MB - pdf)
Datasheets
file_download Ives Single-use MR Conditional Cup Electrodes with Disposable Harness Cable Datasheet
(652.08 KB - pdf)
(652.08 KB - pdf)
Supplementary Information (8)
file_download Ambu Harness Cleaning Instructions
(123.43 KB - pdf)
(123.43 KB - pdf)
file_download Reach document for Medical Devices
(292.55 KB - pdf)
(292.55 KB - pdf)
file_download Ambu Head Diagram, DCPE-21B
(1.31 MB - pdf)
(1.31 MB - pdf)
file_download Ambu Head Diagram, DCPE-23B
(1.42 MB - pdf)
(1.42 MB - pdf)
file_download Ambu Head Diagram, DCPE-25B
(1.42 MB - pdf)
(1.42 MB - pdf)
file_download Ambu Head Diagram, DCPE-27B
(1.42 MB - pdf)
(1.42 MB - pdf)
file_download Symbol Explanation
(2.31 MB - pdf)
(2.31 MB - pdf)
file_download Rationale for Single patient use devices
(606.22 KB - pdf)
(606.22 KB - pdf)
April 2020