aScope™ Duodeno 2
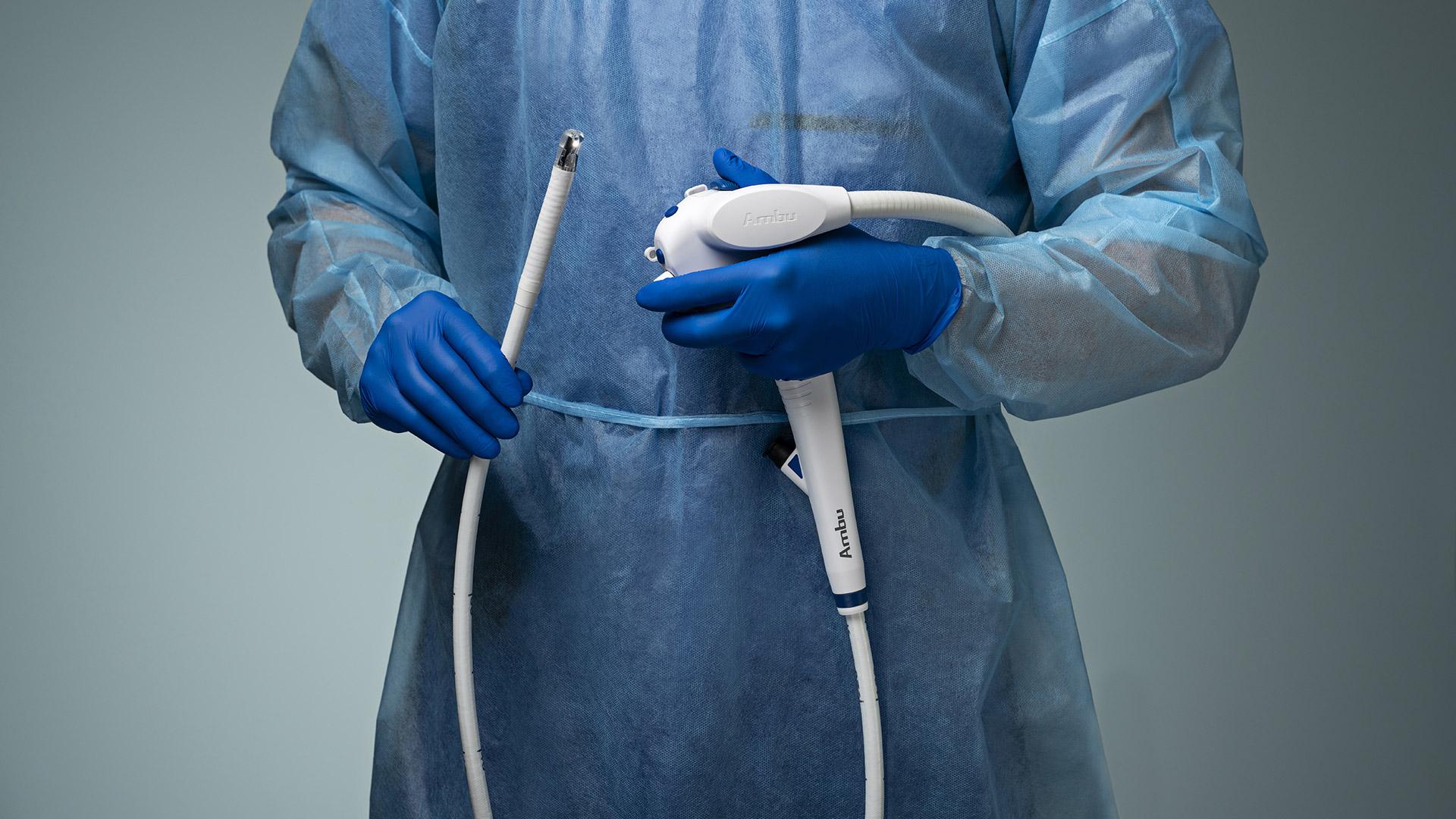
Designed to Fit Your Workflow, Not Disrupt It
Ambu aScope Duodeno™ 2 is a sterile, single-use Duodenoscope which complements your existing ERCP workflow. It was developed in collaboration with leading gastroenterologists to meet the high-quality performance standards required.
An extra measure of safety
As a sterile solution, aScope Duodeno 2 provides an extra measure of safety for your more vulnerable patients.
Always ready - anytime, anywhere
Whether it’s for weekend cases, end of the day add-ons, or procedures outside the suite, the aScope Duodeno 2 gives you a portable, always-available solution to optimize workflow and make the most of your resources.

